Mechanical & Flow Environment within Solid Tumors
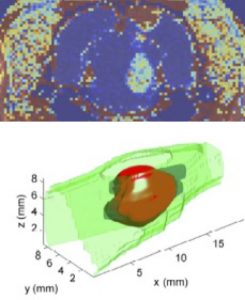 Uneven drug coverage is a major problem encountered in chemotherapy since the clinical goal is to eliminate 100% of tumor cells. Our lab is developing theoretical mechanics models of solid tumors where leaky vessels and cell proliferation contribute to abnormal flow and tissue deformation patterns that adversely affect drug delivery. We use a new magnetic resonance imaging (MRI)-based approach for computational modeling (voxelized modeling method) that uniquely accounts for underlying vascular leakiness as determined by leakage of contrast agent as measured by MRI (dynamic-contrast enhanced MRI, DCE-MRI). This modeling approach accounts for heterogeneous deposition, elevated interstitial fluid pressures, and abnormal tissue flows and provides a major advance towards patient-specific tumor treatment. Currently, we are working with Dr. James Ewing (Henry Ford Hospital) to develop DCE-MRI based models of soft glioma and surrounding brain tissue.
Uneven drug coverage is a major problem encountered in chemotherapy since the clinical goal is to eliminate 100% of tumor cells. Our lab is developing theoretical mechanics models of solid tumors where leaky vessels and cell proliferation contribute to abnormal flow and tissue deformation patterns that adversely affect drug delivery. We use a new magnetic resonance imaging (MRI)-based approach for computational modeling (voxelized modeling method) that uniquely accounts for underlying vascular leakiness as determined by leakage of contrast agent as measured by MRI (dynamic-contrast enhanced MRI, DCE-MRI). This modeling approach accounts for heterogeneous deposition, elevated interstitial fluid pressures, and abnormal tissue flows and provides a major advance towards patient-specific tumor treatment. Currently, we are working with Dr. James Ewing (Henry Ford Hospital) to develop DCE-MRI based models of soft glioma and surrounding brain tissue.
Flow and Drug Delivery in the Brain & Spinal Cord
 Many promising therapeutic agents including tumor-targeting compounds, gene vectors, and nanoparticles are large molecules for which poor tissue penetration continues to be a major problem. Our lab has developed new computational models that use fiber track information embedded in MR-based diffusion tensor imaging data to account for paths of least resistance to flow. We then generate 3D MRI-based computational models to predict infusion patterns in complex brain structures. These models are new tools that allow for personalized therapy of neurological diseases such as epilepsy, Parkinson’s disease and brain tumors. These studies are in collaboration with Drs. Thomas Mareci (UF Molecular Cell Biology) and Paul Carney (UNC Pediatrics). This research (NIH R21 and RO1 funded) has resulted in numerous academic journal articles (>20), 1 patent, and the training of many graduate students.
Many promising therapeutic agents including tumor-targeting compounds, gene vectors, and nanoparticles are large molecules for which poor tissue penetration continues to be a major problem. Our lab has developed new computational models that use fiber track information embedded in MR-based diffusion tensor imaging data to account for paths of least resistance to flow. We then generate 3D MRI-based computational models to predict infusion patterns in complex brain structures. These models are new tools that allow for personalized therapy of neurological diseases such as epilepsy, Parkinson’s disease and brain tumors. These studies are in collaboration with Drs. Thomas Mareci (UF Molecular Cell Biology) and Paul Carney (UNC Pediatrics). This research (NIH R21 and RO1 funded) has resulted in numerous academic journal articles (>20), 1 patent, and the training of many graduate students.
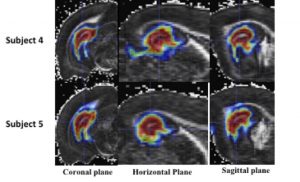
More recently, my lab has conducted tracer studies that focus on uptake along perivascular spaces (PVS) in the brain. These fine features are thought to play an important role in brain clearance, e.g. glymphatic system, which is important for understanding disorders such as Alzheimer’s disease. We have been able to show PVS connections between ventricles and different parts of the brain suggesting a possible role for ventricles as a source or sink for solutes in the brain.
Movie caption. Coronal, sagittal, and horizontal maximum intensity projections of contiguous regions of interest, spanning 31 voxels in the projected direction, for the tracer infused whole rat brain perivascular network registered to the template rat brain atlas. Magdoom et al In Press
Mechanical Characterization of Soft Brain Tissue
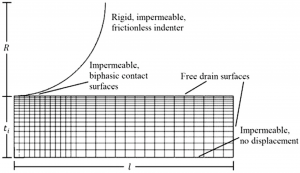 Within very soft tissues, mechanical behavior is often dependent on the interplay of fluid and solid tissue constituents that include cells, extracellular matrix, and vasculature. Our lab has developed micro-indentation methods for measuring very soft mechanical properties of live brain tissue slices in which fluid-filled extracellular spaces are maintained. These spaces are important to maintain since extracellular spaces disappear with swelling after cell death. These experimental studies are in collaboration with Drs. Huikai Xie (UF ECE) and Ghatu Subhash (UF MAE). We are also modeling the mechanical contributions of vasculature and/or axonal fibers as a fiber network in brain tissues.
Within very soft tissues, mechanical behavior is often dependent on the interplay of fluid and solid tissue constituents that include cells, extracellular matrix, and vasculature. Our lab has developed micro-indentation methods for measuring very soft mechanical properties of live brain tissue slices in which fluid-filled extracellular spaces are maintained. These spaces are important to maintain since extracellular spaces disappear with swelling after cell death. These experimental studies are in collaboration with Drs. Huikai Xie (UF ECE) and Ghatu Subhash (UF MAE). We are also modeling the mechanical contributions of vasculature and/or axonal fibers as a fiber network in brain tissues.
Blast traumatic brain injury
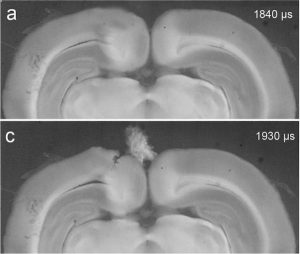 Exposure to explosive blasts can produce complex brain pathology and debilitating functional outcomes collectively termed as blast-induced traumatic brain injury (bTBI). Our lab has studied the direct interaction of the blast wave within brain tissues, as well as, the formation and collapse of bubbles i.e. cavitation. Cavitation events can produce enormous localized strains and pressures which may have consequences for injury. We are one of few labs testing the effect of brain injury due to this effect. These studies are in collaboration with Drs. Ghatu Subhash (UF MAE) and Mike King (UF, Pharmacy).
Exposure to explosive blasts can produce complex brain pathology and debilitating functional outcomes collectively termed as blast-induced traumatic brain injury (bTBI). Our lab has studied the direct interaction of the blast wave within brain tissues, as well as, the formation and collapse of bubbles i.e. cavitation. Cavitation events can produce enormous localized strains and pressures which may have consequences for injury. We are one of few labs testing the effect of brain injury due to this effect. These studies are in collaboration with Drs. Ghatu Subhash (UF MAE) and Mike King (UF, Pharmacy).
Contractility-induced Residual Stresses
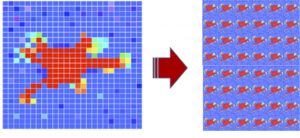 Tissue engineering and regenerative medicine hold the promise of rebuilding organs for transplants and reconstructing diseased tissues. These engineered tissues typically include millions of contracting cells that collectively generate residual stresses. We are using Raman spectroscopy in a novel application to monitor mechanical changes in scaffolds with the introduction of cells. Our lab is developing computational bio-composite models that quantify the contributions of cell contraction to the constitutive response of engineered tissues. This is a NSF funded project that is in collaboration with Drs. Ghatu Subash (UF MAE) and Chelsey Simmons (UF MAE).
Tissue engineering and regenerative medicine hold the promise of rebuilding organs for transplants and reconstructing diseased tissues. These engineered tissues typically include millions of contracting cells that collectively generate residual stresses. We are using Raman spectroscopy in a novel application to monitor mechanical changes in scaffolds with the introduction of cells. Our lab is developing computational bio-composite models that quantify the contributions of cell contraction to the constitutive response of engineered tissues. This is a NSF funded project that is in collaboration with Drs. Ghatu Subash (UF MAE) and Chelsey Simmons (UF MAE).
