Probing the Effect of Ion Insertion on the Mechanical Stability of High Capacity Nanocomposite Anodes
To fully understand the mechanical behavior of nanocomposite anodes an interdisciplinary approach is followed which combines detailed electrochemical experiments along with multiphysics modeling. The specific focus is to determine the effect of lithium-ion (or sodium-ion) insertion and de-insertion on silicon and/or tin nanoparticles coated with polymers. A new computational model will be formulated and implemented, which will allow for the prediction of the appropriate combination of particle size and polymer coating that will inhibit damage at the interfaces of the nanocomposite anodes during battery operation. Based on the theoretical predictions new anodes will be fabricated and tested. Particularly high-resolution electron microscopy will be able to capture the extent of damage at the particle-polymer interface and verify the model. After experimental validation, design guidance will be available for battery developers to fabricate next generation electrodes for both lithium and sodium ion batteries.
Summary of recent results
- Damage Formation in Sn Film Anodes of Na-Ion Batteries, Li, Gulzar, Proietti Zaccaria, Capiglia, Hackney, Aifantis. The Journal of Physical Chemistry C 2019 123 (24), 15244-15250
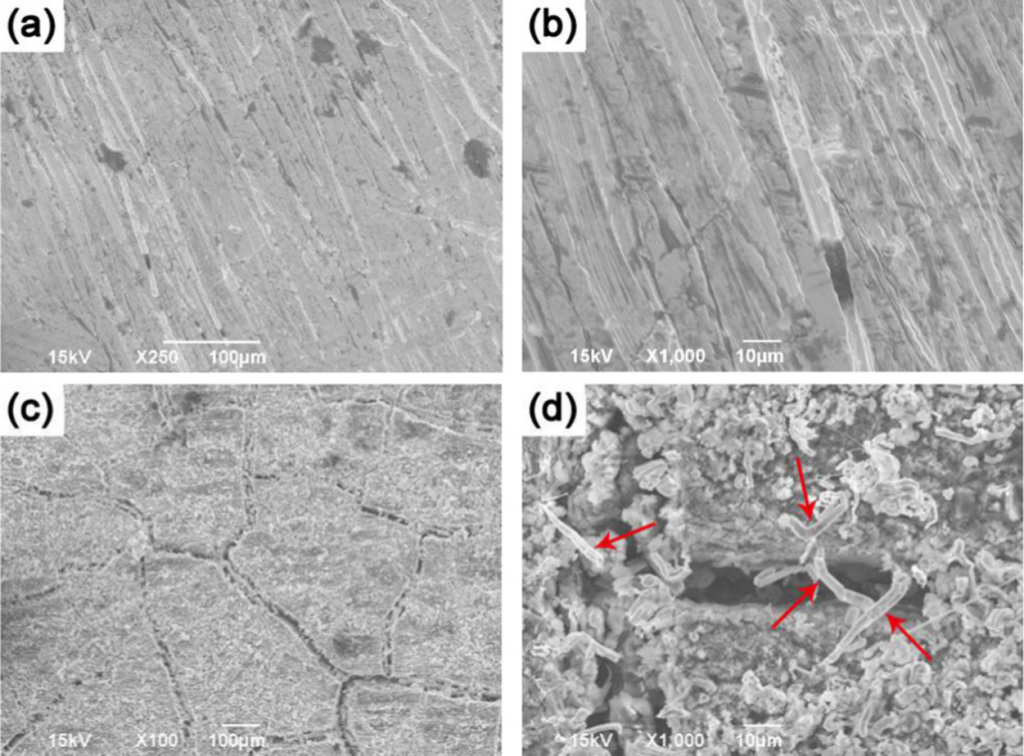
Sn anodes for Na-ion batteries exhibit a promising initial capacity of 847 mAh g–1, which however, cannot be retained throughout continuous cycling due to the 420% volume changes that Sn experiences during sodiation. Previous experimental studies suggest that fracture does not occur in the submicron Sn particles during the formation of Na-Sn alloys; however, such colossal volume changes must result in microstructural damage. In the present work, the damage mechanisms during sodiation are isolated and accentuated by employing a Sn thick film of 0.5 mm as the anode. This simplified planar geometry allows to dispense with the influence of the binder and carbon additives that are required in porous electrodes. Post-mortem electron microscopy revealed new deformation mechanisms for anode materials, as multiple whiskers nucleated on the surface of the Sn, whereas pores formed within the Sn (over the Na-ion penetration distance) after electrochemical cycling. These mechanisms were in addition to the dry lake-bed fracture that was also observed. A comparative study on a Sn thin-film anode of 0.06 mm revealed the formation of fracture and pores after cycling, but no whiskers. The whiskers and pores observed in the thick Sn film anode may be more subtle at the nanoscale, and therefore have not been reported for submicron Sn particles in porous electrodes during sodiation.
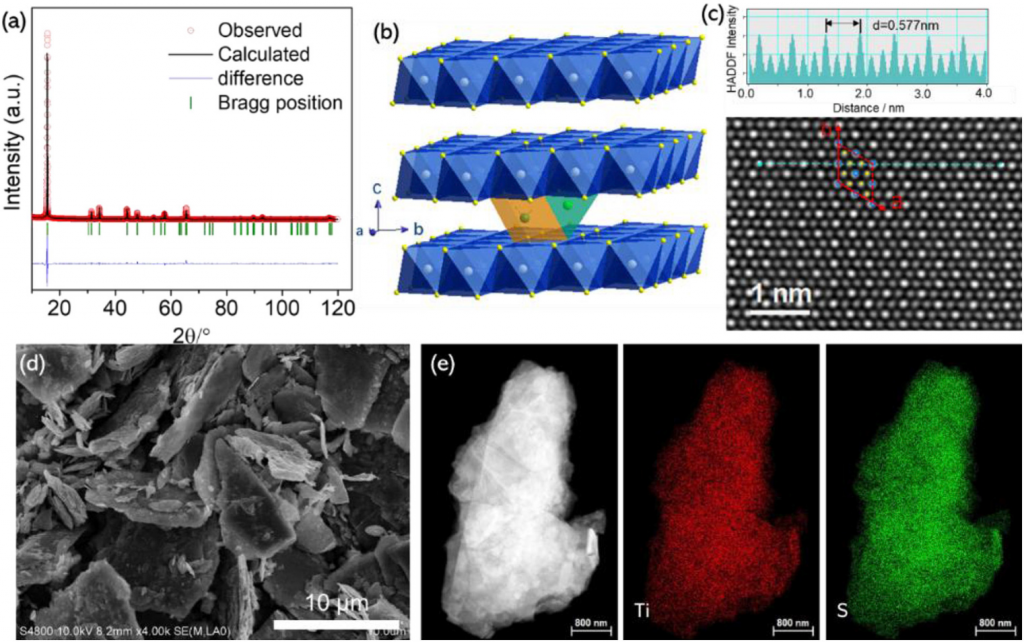
- Capturing the differences between lithiation and sodiation of nanostructured TiS2 electrodes, Hu, Wang, Xiao, Aifantis. Nano Energy, Volume 63, 2019, 103820, ISSN 2211-2855,
In this study TiS2 is chosen as a model electrode material to investigate the relationship between the electrochemical and mechanical performance of layered cathodes for Na-ion batteries. Employing NaFP6 in EC/DMC as the electrolyte allowed for the most promising electrochemical properties recorded in the literature, namely a reversible capacity of 203 mAh g−1 at 0.2 C and 88 mAh g−1 at 10 C with a capacity retention of 92% over 50 cycles. Despite this promising performance the capacity still decayed during long term cycling. In-situ x-ray diffraction and high-resolution transmission electron microscopy imaging revealed that TiS2 underwent a large expansion of 17.7% along the c direction and irreversible phase transformations took place during the sodiation/de-sodiation process, which lead to severe mechanical strains and intragranular cracks. In comparison, the mechanical stability of TiS2 in Li-ion cells was significantly higher. The experimental results are interpreted within a continuum mechanics model which revealed that the maximum effective von Mises stress that is present at the interface between the ion-intercalated TiS2 and pristine TiS2 is about four times higher during sodiation than lithiation indicating that the electrode is more susceptible to failure/fracture during sodiation.