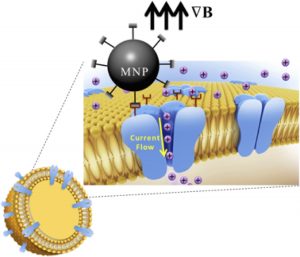
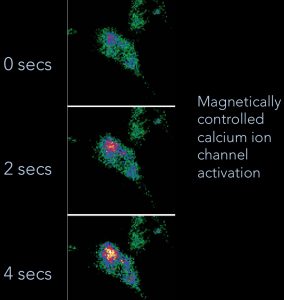
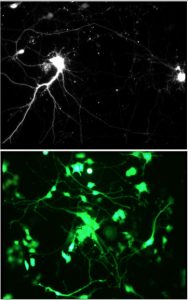
Magnetic Activation of Receptor Signaling (MARS) / Magnetic Ion Channel Activation (MICA)We have been developing the theoretical and experimental framework for MARS/MICA since 1995. These novel technologies enable remote of control cell signaling with magnetic nanoparticles for applications in tissue engineering, regenerative medicine, wound healing, and drug discovery. MARS/MICA consists of two primary approaches. In the first approach, magnetic nanoparticles are decorated with targeting molecules and bind to cell surface receptors. Energy is transferred from an external applied magnetic field to the particles, inducing a conformational change in the receptor, activating the associated molecular signaling pathway. In the second approach, we are using magnetic nanoparticles to control the activation of signaling molecules such as TGF-beta. Latent TGF-beta is conjugated to magnetic nanoparticles and the transfer of energy from the field to the particle changes the latent complex from ‘closed’ (sequestering active TGF-beta) to ‘open’ (releasing active TGF-beta). Collaborators Prof. Alicia El Haj – Keele University References
|
|
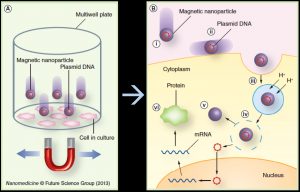
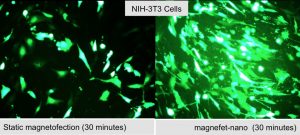
Magnetic Nanoparticle-Based Gene TransfectionWith the sequencing of the human genome and the advent of gene therapy has come the need to develop effective delivery and transfection agents. These agents must be able to target therapeutic and reporter genes to the relevant cells and organs both in vitro for basic investigations as well as in vivo for therapeutic applications. Safety concerns over the use of viral vectors have begun to shift the emphasis toward the development of non-viral delivery agents, primarily cationic lipids. Our group has been working on the development of a novel magnetic nanoparticle-based gene transfection systems based on oscillating arrays of magnets. In these “magnefect” systems, DNA or siRNA is attached to magnetic nanoparticles via charge interactions with the positive, polymer-coated nanoparticle surface. Oscillating arrays of magnets placed underneath a cell culture plate (in the case of in vitro transfection) are used to stimulate particle uptake and endosomal escape via the particle motion, improving gene expression or, in the case of siRNA, knockdown. In addition, we are developing novel, high-gradient magnet arrays and new motion control systems to improve efficiency. Our in vivo work in this area has focused not only on the delivery of nanoparticle/drug/gene complexes but also cells. The aim of this work is to load cells with biocompatible magnetic nanoparticles and re-introduce them into the body, using high-gradient magnets to target them to repair sites or tumours. We have successfully enhanced the natural tumour homing ability of human macrophages by loading them with magnetic nanoparticle/reporter gene complexes. The in vivo uptake of these “therapeutically armed” cells into the non-vascularized, hypoxic cores of solid tumours was enhanced by more than three-fold over non-magnetized cells. We have also used the technology to target human mesenchymal stem cells to tissue repair sites in small animal models. Collaborators Prof. Chris Batich – University of Florida Prof. Claire Lewis & Dr. Munitta Muthana – Sheffield University Prof. Helen Byrne – Oxford University Dr. Jenson Lim – University of Stirling (Scotland) References
|
|
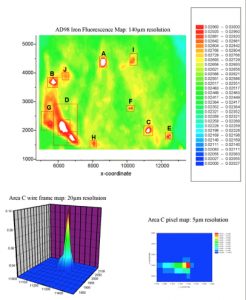
Brain Iron and Neurodegenerative DiseaseOver the past 15 years our group has pioneered synchrotron x-ray and Superconducting Quantum Interference Device (SQUID) magnetomoetry-based detection techniques in order to quantify, characterize and map specific iron compounds in neurodegenerative tissue. We have identified and characterized previously unknown iron compounds associated with Alzheimer’s disease and Parkinson’s disease and have shown that iron accumulation related to neurodegenerative disease is inhomogeneous within diseased tissue, and is associated with specific disease structures, such as AD plaques. In 2001, we were the first to propose that mishandling of iron by ferritin may result in the formation of magnetic iron oxides such as magnetite in these diseases, and that those iron oxides may be detectable via MRI as early biomarkers of AD. We have also shown that iron and mis-folded AD proteins interact in a way that produces magnetic iron compounds. This work aims to provide a better understanding of the role of disrupted iron homeostasis in neurodegenerative diseases and to guide the development of chelation therapies. More recently, data from these studies has been used by us and other groups in the continuing development of MRI-based diagnostic techniques, which aim to use iron compounds formed due to neurodegenerative diseases as a biomarker. Collaborators Dr. Neil Telling – Keele University Prof. Quentin Pankhurst – University College London Dr. Joanna Collingwood – Warwick University References
|
|
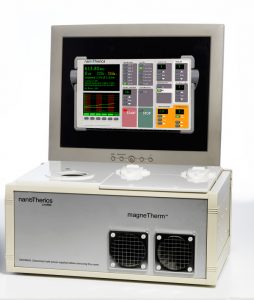
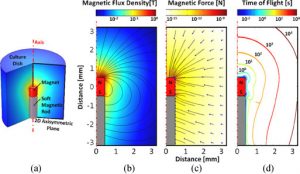
Device DesignTo support our experimental work on biomedical applications of magnetic particles, our lab has been active in designing and developing new hardware for these applications. Devices such as our MICA Magnetic Force Bioreactor for MARS/MICA work, the magneTherm radiofrequency coil system, and the magnefect-nano MNP-based transfection system have formed the foundation of three spin-off companies commercializing technology from our lab – nanoTherics, MICA BioSystems, and 42Bio. We are also designing novel, high-gradient magnet arrays and systems for magnetic separation and targeting applications. Through the commercialization of this technology, these devices are now in use in dozens of laboratories around the world. Collaborators Dr. Neil Farrow – Nanoscience Laboratories (UK) Prof. Chris Batich – University of Florida Prof. Peter McFetridge – University of Florida Dr. Mark Davidson – Tech Toybox Prof. Alicia El Haj – Keele University Patents
|
|
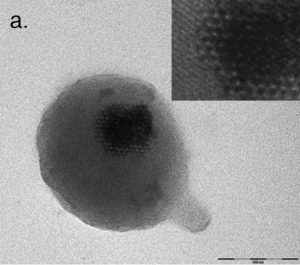
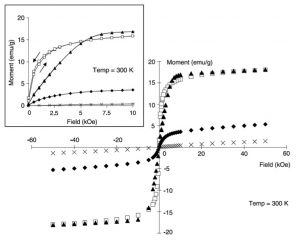
Magnetic Micro- and Nanoparticle Synthesis & FunctionalizationOur group is also active in the development of techniques for the synthesis of novel magnetic nanoparticles and their surface functionalization for, and performance in, biomedical applications. This work focuses on producing particles with enhanced magnetic properties and/or surface chemistry for cell receptor targeting, DNA/siRNA loading, and MRI contrast. I originally proposed the use of w/o microemulsion for producing iron oxide nanoparticle cores with tailored silica shell coatings. These particles were synthesized by colleagues Dr. Weihong Tan and Dr. Swadeshmukul Santra and the results were published in Langmuir. In addition, we synthesized and characterized early versions of dextran and PVA-coated iron oxides and developed particles for cell receptor targeting and DNA transfection. These particles have been characterized via a variety of techniques including High-Resolution Transmission Electron Microscopy and Superconducting Quantum Interference Device (SQUID) magnetometry. Collaborators Prof. Peter McFetridge – University of Florida Prof. Carlos Rinaldi – University of Florida Prof. Blanka Sharma – University of Florida Prof. Tim St. Pierre – University of Western Australia References
|
|
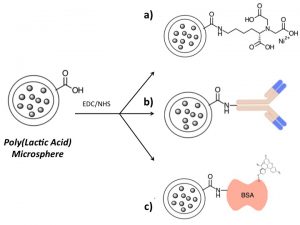
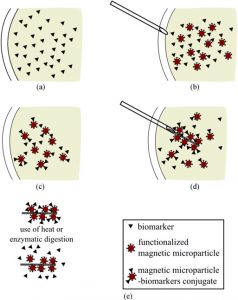
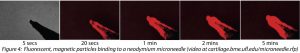
Magnetic Nanoparticle-based Tools for OA DiagnosisIn collaboration with Prof. Kyle Allen, we are developing a novel magnetic nanoparticle-based technology for collection and analysis of osteoarthritis biomarkers. Using this technology, it is possible to collect OA biomarkers directly from a joint without the need to remove joint fluid. In the future, this technology may enable the collection of diagnostic biomarkers from small joints affected by OA, such as the hand, and allow treatments to be tailored to the specific mediators within a joint. We are also developing the technology as a rapid and simple surrogate measure for synovial fluid viscosity. Collaborators Prof. Kyle Allen – University of Florida Prof. Carlos Rinaldi – University of Florida Prof. David Arnold – University of Florida References
|
|