Pioneering Precision in Drug Delivery, Immunotherapy, and Cell-Based Solutions
Targeted Drug Delivery
Despite advances in drug discovery and cell-based products, effective therapies for osteoarthritis (OA) remain elusive. OA is highly complex degenerative joint disease with maladaptive processes occurring within different tissues in the joint (eg. cartilage, synovium, and bone), each with their own unique drug delivery barriers and considerations. The Sharma Laboratory seeks to overcome barriers to effective drug delivery within the joint, by exploiting specific nanoparticle-extracellular matrix interactions and nanoparticle-cell interactions to improve tissue targeting and retention of therapeutic molecules within OA joints.

Immunomodulation in Osteoarthritis
Immunomodulation in osteoarthritis (OA) refers to the process of regulating the immune system’s response to reduce inflammation, slow disease progression, and promote tissue repair. OA is traditionally seen as a degenerative joint disease driven by mechanical wear and tear, but immune system involvement—particularly chronic inflammation—plays a key role in its pathology. The Sharma Laboratory is employing novel materials to target the immune cells and signaling pathways that contribute to the inflammatory environment in affected joints.
Cell-Based Therapies
A hallmark of cancer is escape from immune surveillance. Therapies aimed at reactivating the immune system have seen promising results in certain types of cancer, but there remains high variability in response rates and serious safety issues. Thus, there is an emerging interest in developing therapies that enhance the ability of the body’s own natural killer (NK) cells, which are immune cells that have an inherent ability to recognize cancer cells and destroy them without the safety issues associated with current therapies. A critical hurdle associated with NK cell therapies for solid tumors is poor tumor infiltration and cytotoxic function in the tumor microenvironment. The Sharma laboratory seeks to understand how the tumor microenvironment impacts NK cell migration and activation, and develop strategies to overcome NK cell immunosuppression to destroy tumors.
On-going work
Magnetic particle imaging for nanoparticle tracking
Numerous drugs are currently under investigation as osteoarthritis (OA) therapeutics, but there is a lack of effective delivery systems to improve therapeutic retention and efficacy. Nanoparticles are a promising tool to improve intra-articular drug delivery. It is important to characterize how nanoparticle behave after intra-articular delivery to the joint. Current techniques to quantify and track nanoparticles in vivo are limited. This results in inadequate understanding of how nanoparticles behave, specifically in terms of its retention, clearance, and localization to the joint. To address this limitation, this project focus on engineering magnetic nanoparticles with targeting properties to joint cartilage. Its magnetic properties allow for utilization of an emerging imaging modality- Magnetic Particle Imaging (MPI) to effectively track and assess how NPs behave in the joint. With the application of MPI, we can effectively understand particle fate over time, which is important for attaining insights about drug profiles, and developing strategies to improve therapeutic impact.


Scavenging for reactive oxygen species in osteoarthritis
Joint injuries often lead to increased presence of reactive oxidative species (ROS) within the joint due to chronic increases in mechanical stress on cells. When intrinsic antioxidant mechanisms are not able to compensate for increased presence of ROS, oxidative stress occurs. The products of oxidative stress, including oxidation of proteins, degradation products,cell death and release of cellular contents, leads to stimulation of inflammatory cytokines by cells in the joint. Bioactive nanoparticles made of manganese dioxide (MnO2) are being formulated in the Sharma Lab to penetrate into cartilage and demonstrate prolonged joint retention. These nanoparticles act as both drug delivery system and therapy as a treatment for post traumatic osteoarthritis. The nanoparticles are being tested with an in vitro cytokine-challenged cartilage model and an in vivo disease model and evaluated for providing protection from inflammation-induced oxidative stress.
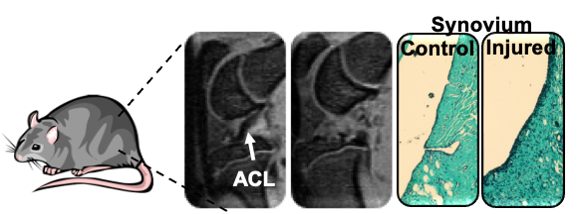
Animal models to advance understanding of PTOA progression
The use of animal models serves as critical pre-clinical tool to study the relationship between disease pathogenesis and related OA symptoms. Common animal model of OA relies on surgical methods to initiate OA. However, these models lack clinical translation and fail to capture the mechanical trauma to the joint, that induces post traumatic osteoarthritis (PTOA). Being said, this project focus on the development and characterization of a non-invasive knee injury model (NIKI) of PTOA. This is achieved by characterization of animal behavior via gait analysis (in collaboration with Dr. Kyle Allen), inflammatory makers, and evaluation joint modeling changes via histology and bone morphology changes.

Investigating natural killer cell function in engineered 3D tumor models
NK cells are among the first-responder immune cells that recognize abnormal cells and destroy them. However, solid tumors establish a complex ecosystem, known as the tumor microenvironment, that suppress NK cell functions. The tumor microenvironment can alter both cancer cells and immune cell behavior; however, further investigation is needed to understand whether aspects of the tumor microenvironment act as a friend or a foe. We seek to understand the mechanisms by which the tumor microenvironment impacts the homing and anti-tumor activity of NK cells.
Probing mechanisms driving natural killer cell migration
NK cells have emerged as powerful tool for immunotherapy, as they rapidly recognize and lyse cancer cells without prior antigen priming. However, a critical hurdle with NK cell therapies is inadequate infiltration and activation in the solid tumor microenvironment. Yet, factors regulating NK cell tumor infiltration remain largely unknown. The long-term goal of this research is to develop biomimetic models of the solid tumor microenvironment that allow the quantification of NK cell migration and uncover mechanisms of NK cell infiltration into solid tumors.


Using nanoparticle targeted delivery to activate natural killer cells
With the growing interest in utilizing biomaterials to improve cancer immunotherapies, this project is focused on exploring how nanoparticles are taken up by immune cells. NK cells can innately target and kill tumor cells, but face barriers in solid tumors that nanoparticles are able to help them overcome. Looking at how these nanoparticles are taken up can help us develop new nanoparticle systems to improve cell-based immunotherapy outcomes.
Recapitulating aspects of the tumor microenvironment
Currently 2D preclinical cancer models lack interactions that are crucial for tumor development and progression. The need for 3D cancer models is evident in order to have more biologically relevant systems for drug development. We are tissue engineering 3D models of the tumor microenvironment to interrogate how specific biochemical and mechanical cues impact NK cell migration and interaction with cancer cells.


Guiding stem cells to produce cartilage
Despite several decades of research, there are no effective clinical treatment for damaged cartilage in your joints. The underlying mechanisms of attracting stem cells and getting them to turn into the desired cartilage cells are not well understood. I am focusing on investigating the mechanism of stem cell recruitment and chondrogenesis. We are using functionalized polymeric hydrogels and viral reporter constructs in order to track chondrogenic differentiation of mesenchymal stem cells.
Delivery of immunomodulating proteins from microparticles to inhibit inflammation
Synovial macrophages are innate immune cells that play a pivotal role in the progression of osteoarthritis (OA). When activated to a pro-inflammatory state by cartilage degradation products, macrophages produce pro-inflammatory cytokines such as IL-1 and TNF-α. These cytokines drive OA synovitis and influence the production of other pro- and anti-inflammatory cytokines, production of MMPs, and expression of aggrecanases in the OA synovium. A drug delivery system designed to polarize synovial macrophages from a pro-inflammatory to anti-inflammatory state will inhibit production of pro-inflammatory cytokines and increase presence of anti-inflammatory cytokines within the joint. CD200 is an endogenous membrane glycoprotein that has been shown to deliver an inhibitory signal to the macrophage lineage. PLGA microspheres that encapsulate CD200 have been formulated in the Sharma Lab to target activated macrophages in vitro. By delivering CD200 in PLGA microparticles through intra-articular injection to the OA joint, we hope to (1) retain CD200 in the joint and (2) provide long-term release of CD200. In doing so, CD200 will be able to reduce inflammation long-term and reduce the number of injections needed to maintain therapeutic effect.


ROS-scavenging nanoparticles to mitigate macrophage inflammatory signaling
Macrophages are innate mediators of inflammation and wound healing. Further, macrophages can express different phenotypes, either skewed towards pro-inflammatory or anti-inflammatory characteristics, in response to different stimuli. Classically activated, pro-inflammatory, macrophages have been implicated in OA for their contributions to the inflammatory profile of the joint through cytokine and reactive oxygen species expression. We are investigating the potential of manganese dioxide (MnO2), a nanomaterial with antioxidant-like activity, to scavenge reactive oxygen species in macrophages, with the potential to mitigate macrophage inflammatory signaling.
