Research
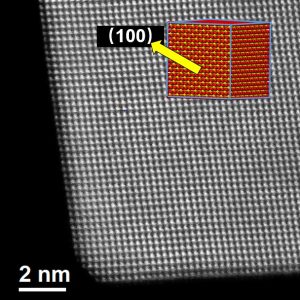
Our research involves the preparation of nanoparticle oxides with narrow size distributions, catalyst preparation using the nanoparticle oxides as supports, and catalyst characterization using several analytical techniques to determine how the particle size of the oxide support influences the active metal and thus also the catalytic activities and selectivities.
The analytical techniques include; Brunauer-Emmett-Teller (BET) surface area measurements, chemisorption of probe molecules, such as carbon monoxide, to determine active metal surface area, temperature programmed reduction and oxidation (TPR and TPO) experiments to determine reduction-oxidation (redox) properties, X-ray photoelectron spectroscopy (XPS) to determine electronic structure and surface chemical composition, transmission electron microscopy (TEM) to determine particle sizes and distribution of active metals on the support, and X-ray diffraction (XRD) measurements to determine crystal structure.
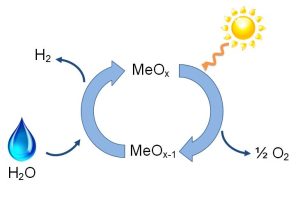
Our main research focus is on environmentally friendly, energy-related reactions. Our projects include catalyst development for hydrogen production via catalytic steam reforming of methanol, C-H activation and C-C coupling of aromatic compounds, oxidative coupling of methane (methane to higher-value chemicals), and thermochemical water-splitting using solar energy.
